
Rendering of a gold molecule.
Unlocking the World of the
Ultrasmall and the Ultrafast
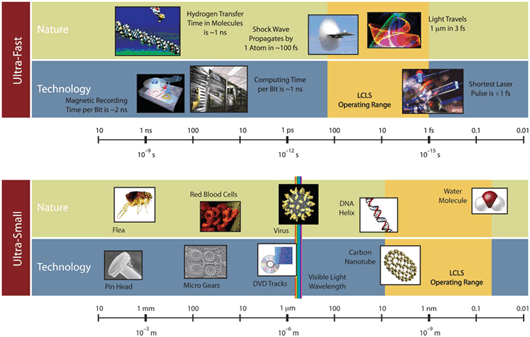
Discovered in 1895 by German physicist Wilhelm Conrad Röntgen, X-rays are a high-energy, short-wavelength form of light that is invisible to the eye. X-ray wavelengths are several thousand times shorter than those of visible light, and are comparable to the dimensions of atoms.
X-rays also can penetrate through many materials that visible light cannot. Since their initial discovery, X-rays have been used in medicine and dentistry to reveal bones and dense tissues. X-rays have also been used in the laboratory to study the atomic structures of materials.
Today's synchrotron “light-source” facilities produce X-rays that are millions of times brighter than medical X-rays. Scientists use these highly focused, intense beams of X-rays to reveal the identity and arrangement of atoms in a wide range of materials. The tiny wavelength of X-rays allows us resolve the arrangement of atoms in metals, semiconductors, ceramics, polymers, catalysts, plastics, and biological molecules.
However, atoms are constantly moving or vibrating, and synchrotron X-ray sources produce long pulses which yield only blurred images of these motions. LCLS is the first source to produce X-rays that are both very intense and clumped into ultrafast pulses.
« Part 1 |
Part 2 |
Part 3 »
Modern X-ray Science
One of the most active areas of X-ray light-source research is protein crystallography. To determine the structure of a protein, scientists obtain a crystalized sample of protein molecules where all the atoms are ordered into a regular pattern. The crystal sample is usually no bigger than a grain of salt.
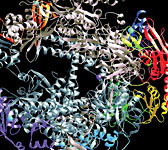
Exposing the crystal to a beam of intense, highly focused X-rays creates a unique pattern of dots on a detector. The spots—called Laue spots—are used to calculate the locations of atoms within the protein molecules.
This technique earned Stanford’s Roger Kornberg the 2006 Nobel Prize in chemistry. Kornberg and colleagues used protein crystallography to solve the structure of RNA polymerase, which contains over 30,000 individual atoms. RNA polymerase plays a key role in how information stored in DNA is translated into the proteins of life.
The LCLS is revolutionizing protein crystallography by making “single-shot” images of molecules that resist forming crystals.



